
If you have any other questions or need other size, please get a quote.
 Zhang, Suhong, et al. Minerals Engineering 179 (2022): 107434.
Zhang, Suhong, et al. Minerals Engineering 179 (2022): 107434.
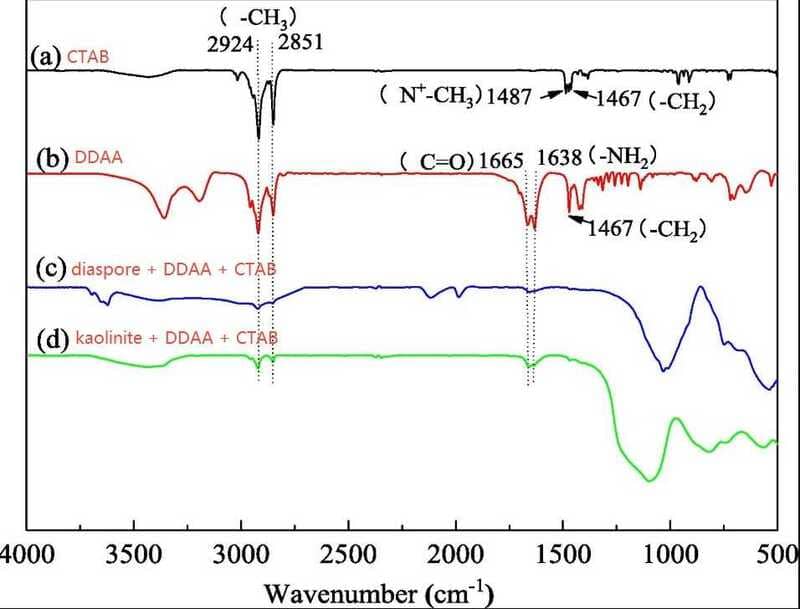
Dodecanamide (DDAA) has been investigated for its role in improving the selective flotation separation of diaspore and kaolinite when used as a mixed collector with cetyltrimethylammonium bromide (CTAB). The flotation experiments demonstrated that DDAA significantly influenced the adsorption behavior of CTAB, thereby enhancing the flotation efficiency of kaolinite while reducing the recovery of diaspore.
Flotation tests on single minerals indicated that diaspore exhibited poor flotability, whereas kaolinite achieved over 92% recovery in the presence of the DDAA-CTAB mixed reagent at pH 5. Artificial mixed mineral flotation further confirmed the improved separation performance, leading to a notable increase in the Al₂O₃/SiO₂ (A/S) ratio in the concentrate. FTIR analysis confirmed the adsorption of both DDAA and CTAB on the mineral surfaces, while zeta potential measurements and XPS analysis showed that the presence of DDAA enhanced the adsorption difference of CTAB between diaspore and kaolinite.
Molecular dynamics simulations provided further insights, revealing that DDAA preferentially adsorbed on diaspore, while CTAB was more strongly adsorbed on kaolinite. This selective adsorption mechanism facilitated an effective separation process, optimizing the flotation of kaolinite while suppressing diaspore recovery.
The study highlights DDAA's role as an auxiliary collector in flotation processes, demonstrating its effectiveness in modifying reagent adsorption behavior and improving mineral separation efficiency.
 Shi, Haodong, et al. Minerals Engineering 225 (2025): 109218.
Shi, Haodong, et al. Minerals Engineering 225 (2025): 109218.
Tetradecyl trimethyl ammonium chloride (TTAC) has emerged as an efficient cationic collector for the selective separation of quartz from gypsum (CaSO₄·2H₂O), significantly enhancing the purity of phosphogypsum (PG). PG, a by-product of wet-process phosphoric acid production, contains quartz impurities that affect its whiteness and industrial usability. The use of TTAC as a flotation reagent offers an effective solution to this issue.
Microflotation experiments demonstrated that TTAC exhibited excellent selectivity for quartz across a broad pH range (2.5-9.5), achieving peak separation efficiency at neutral pH (7.0 ± 0.1). At a low TTAC concentration of 100 mg/L, quartz recovery reached 96%, while gypsum recovery remained as low as 21.5%. Adsorption studies further revealed a maximum adsorption capacity of 12.8 mg/g on quartz, with contact angle measurements indicating a 143% increase in hydrophobicity, from 28.4° to 69.1°.
Mechanistic investigations via zeta potential, FT-IR, and XPS analyses confirmed that TTAC interacts selectively with quartz through electrostatic interactions and hydrogen bonding, while its interaction with gypsum remains minimal. This selective adsorption mechanism ensures efficient quartz removal, thereby improving the quality and industrial applicability of PG.
By facilitating the large-scale purification of PG, TTAC not only enhances its commercial value but also contributes to environmental sustainability by promoting the reuse of this industrial by-product.
 Gao, Minlan, et al. Comptes Rendus. Chimie 22.5 (2019): 355-362.
Gao, Minlan, et al. Comptes Rendus. Chimie 22.5 (2019): 355-362.
Tetradecyl trimethyl ammonium chloride (TTAC), a quaternary ammonium cationic surfactant, has been identified as an efficient corrosion inhibitor for A3 steel in hydrochloric acid (HCl) solutions. In a comparative study of alkyl chain length effects, TTAC demonstrated superior inhibition performance relative to longer-chain surfactants like cetyl trimethyl ammonium chloride (CTAC) and octadecyl trimethyl ammonium chloride (OTAC), but slightly lower efficiency than dodecyl trimethyl ammonium chloride (DTAC).
Experimental weight-loss measurements revealed that at 70 mg/L and 50 °C, TTAC significantly reduced the corrosion rate of A3 steel by forming an adsorbed protective layer. The inhibition efficiency followed the order DTAC > TTAC > CTAC > OTAC, indicating that increasing alkyl chain length reduces corrosion inhibition due to a decrease in adsorbed inhibitor molecules at the same concentration. The adsorption process of TTAC conformed to the Langmuir isotherm model, indicating monolayer adsorption with spontaneous and exothermic characteristics.
Thermodynamic analyses revealed that enthalpy change (ΔH), entropy change (ΔS), and activation energy (Ea) increased with alkyl chain length, further confirming the adsorption-driven mechanism. These findings suggest that TTAC effectively mitigates acid-induced corrosion by adsorbing onto the steel surface, reducing metal dissolution.
Given its high inhibition efficiency and moderate alkyl chain length, TTAC presents a promising corrosion inhibitor for industrial applications where protection of steel infrastructure in acidic environments is essential.